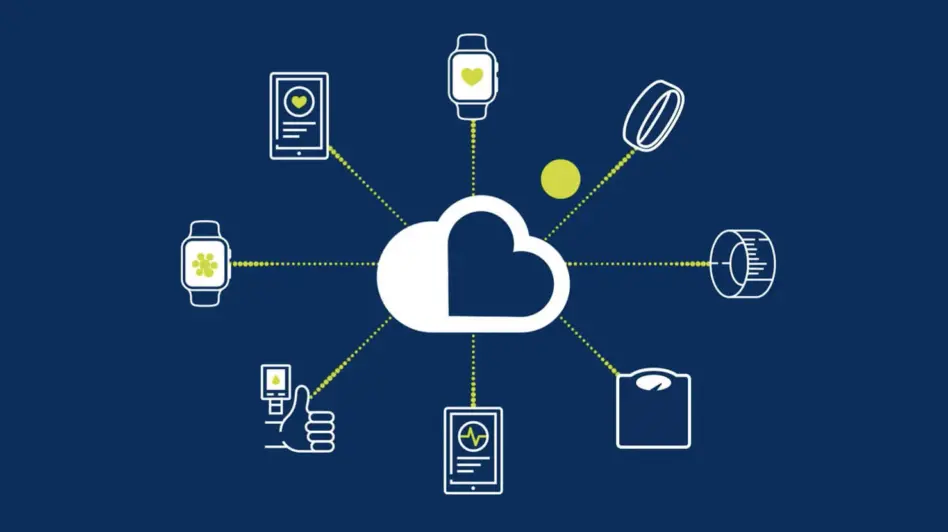
Sensor Cloud
Pioneering the next generation of connected health solutions
Medidata Sensor Cloud provides cutting-edge capabilities focused on transforming the clinical trial experience for patients, sponsors, and research sites. Designed as part of a unified data platform, Sensor Cloud takes a unique approach to managing a broad range of wearable sensor and digital health technology data for clinical trials. Our common data model and proprietary algorithms enable rapid ingestion and analysis of patient data resulting in better clinical decision making, faster timelines and a more flexible patient experience.
The Value of Sensor Data in Clinical Trials

Empowering Patients
Reduce site visits, time, travel and inconvenience through a more flexible approach to trial participation. Wearable sensors and other connected health devices provide non-invasive methods of collecting higher frequency, high fidelity medical grade data that will help to enable new treatments.
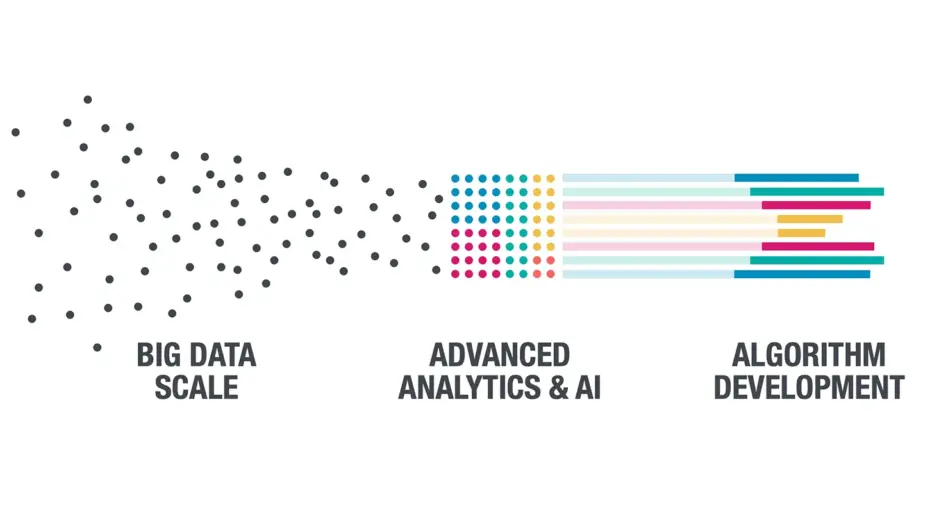
Putting Data into Action
Enable greater insights through proprietary analytics including novel digital biomarker discovery. Our common data model and proprietary algorithms enable better clinical decision making, faster timelines and a more patient-centric experience.

Enhancing Patient Engagement
Identify early compliance indicators and detection of patients’ response to treatment. Efficiencies in the same interfaces, log-ins and workflows reduce time spent on data entry.
Key Features of Sensor Cloud

Rapid Integration
Sensor Cloud delivers seamless integration for all data through a single API connection. Our device library includes a growing list of connected medical grade sensors and devices.
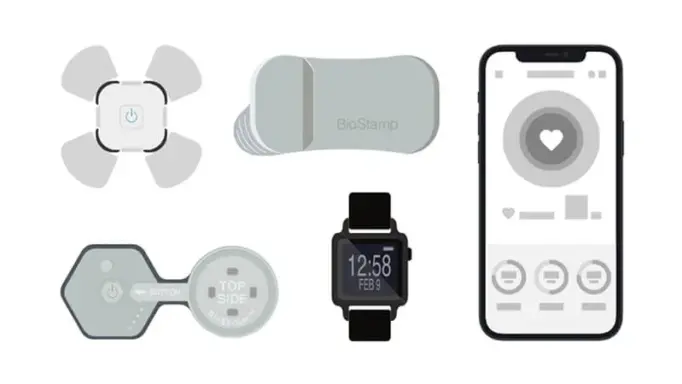
Sensor Library and Scoring
Our experts provide guidance on sensor selection based on rigorous reviews of specifications including therapeutic focus, patient usability, form factor, regulatory status, and other sponsor specific study design criteria.
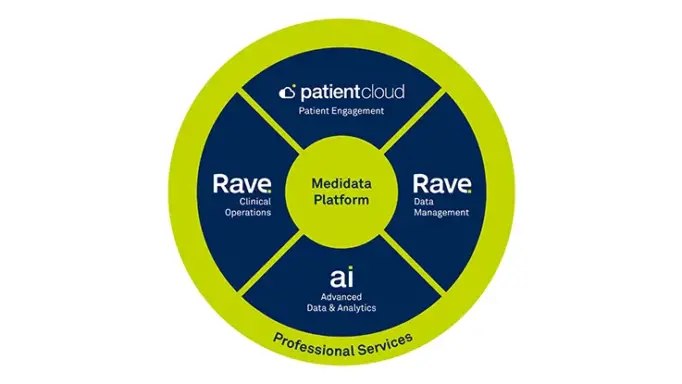
Synergies with the Medidata Platform
Ingest and analyze data from any disparate data source while easily integrating with the Medidata Platform.

Common Data Model
Our common model enables standardization of data resulting in a unified, consistent experience. Ingest, process, normalize, and deliver data from any digital health technology and convert to a common data structure. Data can be directed to the Medidata Platform or to the platform of your choice.
Related Solutions
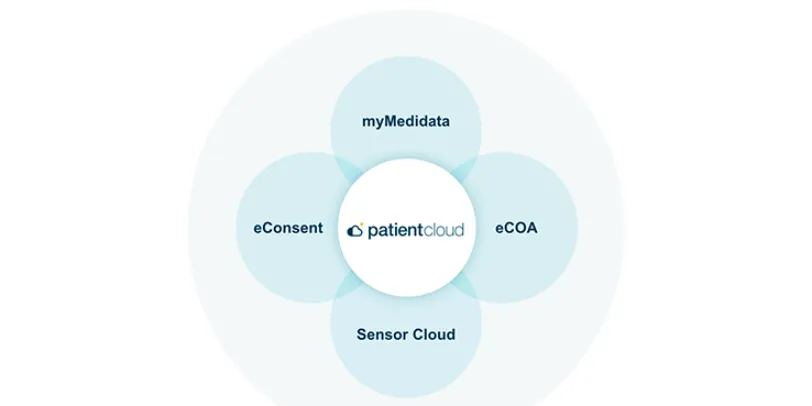
Medidata Patient Cloud
Patient Cloud is a suite of powerful solutions that makes it simple and engaging for patients to participate in any clinical trial – so your trials are easier, faster, and produce better results.
Sensor Cloud Network
Join the brightest minds in data to collaborate, share and solve. As part of the Sensor Cloud Network, you’ll discover new possibilities in sensor integrations, sensor data, digital biomarkers, and algorithms – shaping a better future for us all.
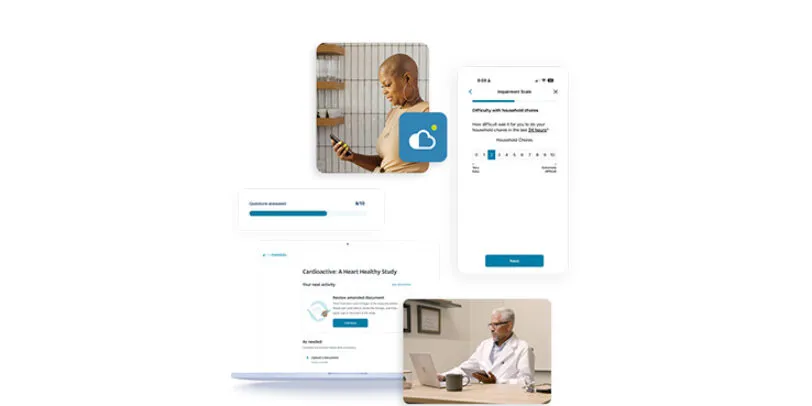
eCOA
eCOA provides a flexible, intuitive model for capturing patient data that is designed to make it easier for patients to engage in clinical trials. Built as part of the unified Medidata Platform, eCOA improves your study experience with flexible deployment options, a groundbreaking global instrument library, and dedicated services and support.
Learn More

Pioneering the Next Generation of Connected Health Solutions
Medidata Sensor Cloud provides cutting-edge data ingestion capabilities focused on transforming the clinical trial experience for patients, sponsors, CROs, and research sites.

Developing Your Mobile Sensor Strategy Journey for Better Study Outcomes
Highlights the key considerations and challenges when implementing a digital health technology strategy for clinical trials.

New Survey Results From ISR and Medidata: Primary Motivations and Challenges With Sensor Usage in Clinical Trials
View our latest infographic highlighting key challenges and motivations for incorporating connected devices into clinical trials.

Advancing Decentralized Clinical Trials through a Unified Approach to eCOA and Digital Health Technologies
Drug developers are increasingly harnessing the combined power of objective data measured using digital health technologies (DHTs) and subjective clinical outcome assessments that include patient-reported outcomes (PROs).
Uniting these two complementary mechanisms is enabling the development of comprehensive patient profiles that better inform how patients experience their disease and respond to treatment.